Embarking on a new clinical trial is an exciting yet incredibly complex endeavor. From recruiting the right patients to securing adequate site resources and navigating intricate regulatory landscapes, there are countless variables that can influence a trial’s success or failure. Before any significant investment of time or capital is made, it’s absolutely critical to conduct a thorough evaluation of the proposed study’s viability.
This is where a well-structured approach to preliminary assessment becomes indispensable. Having a robust system in place to gather essential information about potential sites, patient populations, and logistical challenges can save millions of dollars and years of effort down the line. It’s about proactive problem-solving, identifying hurdles before they become insurmountable roadblocks, and ultimately, setting the stage for a smooth and efficient research journey.
Why a Robust Feasibility Questionnaire is Non-Negotiable
Imagine investing years of research and millions of dollars into a clinical trial, only to discover halfway through that patient recruitment targets are unattainable, or that the chosen sites lack the necessary infrastructure. Such scenarios are not just costly; they can severely damage reputations and delay life-saving treatments. This is precisely why a comprehensive feasibility assessment, guided by a meticulously designed questionnaire, isn’t just a good idea—it’s an absolute necessity. It serves as your early warning system, highlighting potential bottlenecks and risks before they escalate into major problems.
A detailed clinical trial feasibility questionnaire helps you gather critical insights from potential investigative sites, giving you a realistic picture of their capabilities and limitations. It allows you to move beyond assumptions and base your strategic decisions on concrete data. By proactively identifying issues such as low patient prevalence, fierce recruitment competition, or inadequate site staff, you can either adjust your protocol, select more suitable sites, or even determine if the trial is feasible at all, saving invaluable resources and time.
Furthermore, a well-executed feasibility study through such a questionnaire promotes transparency and open communication between sponsors, CROs, and sites. It ensures that everyone involved has a clear understanding of the study’s demands and the resources required. This collaborative approach fosters stronger partnerships and builds a foundation of trust, which is crucial for the long-term success of any clinical research program. It’s about setting realistic expectations and aligning all stakeholders from the outset.
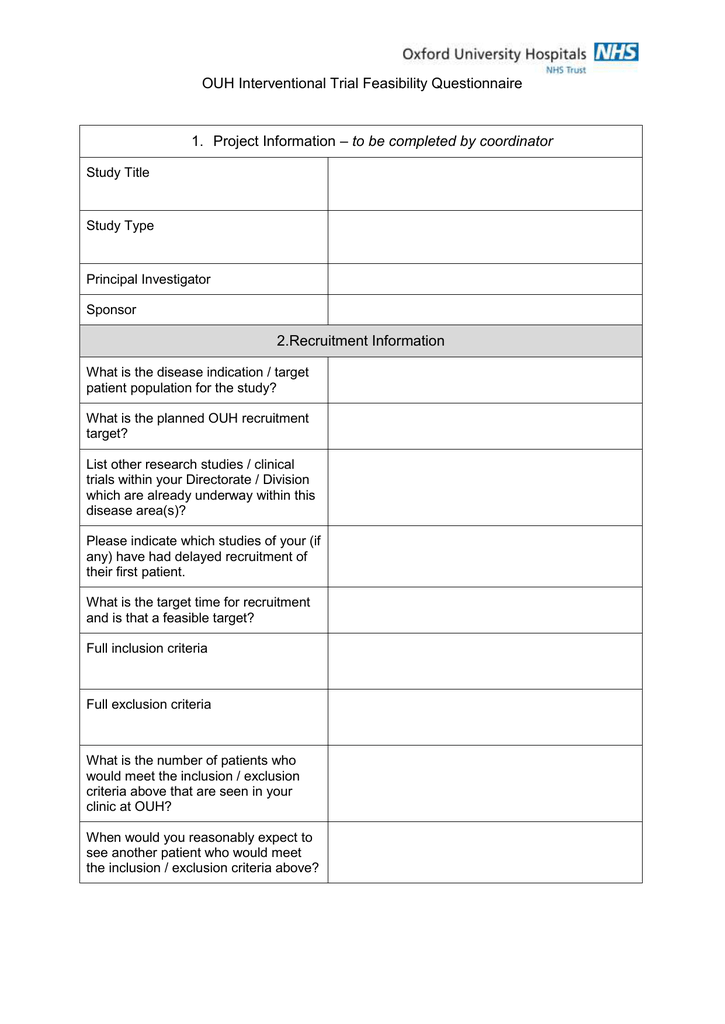
Key Areas Covered in a Comprehensive Feasibility Questionnaire
A truly effective questionnaire delves into multiple facets of a potential trial site and its environment. It goes beyond simple “yes” or “no” questions, seeking detailed qualitative and quantitative data.
- Patient Demographics and Recruitment Potential: Understanding the size and characteristics of the potential patient pool, including prevalence of the disease, current treatments, and competing trials.
- Investigative Site Capabilities: Assessing the site’s experience with similar trials, available staff (investigators, nurses, coordinators), equipment, laboratory facilities, and patient database management systems.
- Regulatory and Ethical Environment: Ensuring the site adheres to local and international regulations, has a functioning Institutional Review Board (IRB) or Ethics Committee (EC), and understands the protocol’s ethical considerations.
- Budget and Contractual Logistics: Determining the site’s willingness to work within budget constraints, their contract negotiation processes, and any specific financial requirements.
- Operational Logistics: Evaluating drug storage capabilities, data entry systems, monitoring visit logistics, and overall site management efficiency.
- Competitive Landscape: Identifying other ongoing or planned trials that might compete for the same patient population or site resources.
By systematically addressing these critical areas, a robust questionnaire provides a holistic view, enabling informed decisions that pave the way for successful trial execution.
Building Your Own Effective Clinical Trial Feasibility Questionnaire Template
While numerous generic templates exist, the true power of a clinical trial feasibility questionnaire template lies in its adaptability and relevance to your specific study. A one-size-fits-all approach rarely yields the most insightful results. Instead, view a template as a foundational framework that needs to be meticulously tailored to the unique demands of your protocol, therapeutic area, and target patient population. This customization ensures that you’re asking the right questions to glean truly actionable intelligence, rather than just collecting generic data.
Start by reviewing your study protocol in detail. What are the most critical inclusion and exclusion criteria? Are there specific procedures, equipment, or drug storage requirements that are unique to your trial? Identifying these unique elements will directly inform the specialized questions you need to add to your template. For instance, a trial involving rare diseases will require very different recruitment questions than a common condition, and a cell therapy trial will demand much more detailed questions about specialized lab capabilities.
The way you phrase your questions significantly impacts the quality of the responses you receive. Ambiguous or leading questions can result in misleading data, while clear, concise, and objective questions elicit the detailed information you need. Consider using a mix of question types: quantitative questions (e.g., “How many eligible patients did your site screen for similar studies last year?”) provide measurable data, while qualitative questions (e.g., “Describe any anticipated challenges in recruiting patients for this study.”) offer valuable contextual insights.
Here are some actionable tips for designing your questionnaire:
- Be Specific: Avoid vague language. Instead of “Do you have lab equipment?”, ask “Do you have a -80°C freezer with temperature monitoring capabilities?”
- Use a Mix of Question Types: Combine yes/no, multiple-choice, rating scales, and open-ended questions to gather both quantitative and qualitative data.
- Consider the Respondent’s Perspective: Frame questions in a way that makes it easy for the site staff to provide accurate information, drawing upon their direct experience.
- Pilot Test Your Questionnaire: Before widely distributing it, test your questionnaire with a few potential sites or internal team members to identify any ambiguities or areas for improvement.
Remember, your clinical trial feasibility questionnaire template is not a static document. It should evolve with your understanding of the clinical research landscape and the specific nuances of your studies. Regularly review and update it based on feedback from sites and the outcomes of your feasibility studies. This iterative process ensures that your questionnaire remains a powerful and effective tool for making informed, strategic decisions that drive successful clinical trials.
Thorough upfront assessment is the cornerstone of successful clinical research, helping to mitigate risks, optimize resource allocation, and accelerate the development of new therapies. By diligently evaluating the myriad factors that influence a trial’s execution, from patient accessibility to site-specific capabilities, research teams can make informed decisions that significantly enhance their probability of success.
This strategic foresight, enabled by systematic inquiry, ensures that trials are not only scientifically sound but also operationally viable. It empowers sponsors and researchers to navigate the complexities of drug development with greater confidence and efficiency, ultimately contributing to the advancement of medical science and delivering much-needed treatments to patients.